Results A 13389G deletion in exon 6 was characterized in propositus, and this mutation led to frameshift.
结果先证者表现为抗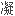

 基
基 外
外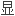
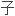 6
6 13389G缺失,引起移码突变。
13389G缺失,引起移码突变。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
In the PROFILE 1001 trial involving 69 patients with MET exon 14-mutant NSCLC (38% treatment naive), the investigator- reported RR was 32%, with median PFS and OS of 7.3 months and 20.5 months, respectively.
在涉及 69 名 MET 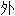 显
显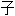 14 突变 NSCLC 患者(38% 未接受过治疗)的 PROFILE 1001 试验中,研究者报告的 RR 为 32%,中位 PFS 和 OS 分别为 7.3
14 突变 NSCLC 患者(38% 未接受过治疗)的 PROFILE 1001 试验中,研究者报告的 RR 为 32%,中位 PFS 和 OS 分别为 7.3 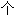 月和 20.5
月和 20.5 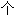 月。
月。
12 Amivantamab became the first targeted therapy to be granted FDA approval for patients with EGFR exon 20 insertion NSCLC in May 2021. Similarly, in September 2021, mobocertinib was approved by the FDA in the same setting.
12 Amivantamab 于 2021 年 5 月成为第
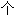 获得 FDA 批准用于治疗 EGFR
获得 FDA 批准用于治疗 EGFR 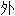 显
显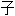 20 插入 NSCLC 患者的靶向疗法。同样,2021 年 9 月,mobocertinib 在相同情况下获得 FDA 批准。
20 插入 NSCLC 患者的靶向疗法。同样,2021 年 9 月,mobocertinib 在相同情况下获得 FDA 批准。
No association was noted between the location or type of the MET exon 14 alteration and outcome; however, the occurrence of co-mutations at baseline in PI3KCA (3%), KRAS (2%) or PTEN (3%) correlated with lack of response to tepotinib.
MET 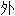 显
显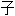 14 改变的位置
14 改变的位置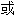 类型与结果之间没有发现关联;然而,PI3KCA (3%)、KRAS (2%)
类型与结果之间没有发现关联;然而,PI3KCA (3%)、KRAS (2%) 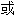 PTEN (3%) 基线时发生的共突变与 tepotinib 缺乏反应相关。
PTEN (3%) 基线时发生的共突变与 tepotinib 缺乏反应相关。