No longer should our legislators be able to publicly excoriate FDA employees while ignoring their own complicity.
国会议员们不应再当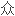
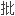
 FDA雇员时而忽略了自己恰恰是同谋犯。
FDA雇员时而忽略了自己恰恰是同谋犯。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
 5/6月合集
5/6月合集  9月合集
9月合集  4月合集
4月合集  四级阅读真题精听
四级阅读真题精听  5月合集
5月合集  源的故事
源的故事  10月合集
10月合集