The benzene ring and alkene structural group are much more stable than structural group of sulfoacid for the carbonifying process of polystyrene.
在碳化过程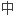 ,
,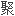
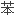 乙
乙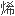 阳离子交换
阳离子交换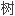 脂的
脂的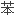 环结构和
环结构和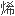
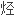
 团比磺酸
团比磺酸 团稳定。
团稳定。
声明:以上例句、词性分类均由互联网资源自动生成,部分未经过人工审核,其表达内容亦不代表本软件的观点;若发现问题,欢迎向我们指正。
 Crash Course
Crash Course  Crash Course
Crash Course  Crash Course
Crash Course  Crash Course
Crash Course  Crash Course
Crash Course  Crash Course
Crash Course  Crash Course
Crash Course  Crash Course
Crash Course  Crash Course
Crash Course  Crash Course
Crash Course